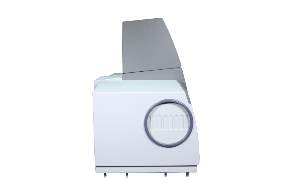
PF7
PF7 Atomic Fluorescence Spectrophotometer is an excellent element analysis instrument which has the advantages of atomic absorption spectrophotometer and atomic emission spectrophotometer but overcomes their shortages in techniques.
The instrument has good accuracy, high sensitivity, simple structure, small volume, and easy operation.
It is designed to test elements that could easily form hydride, gaseous constituents or deoxidizes to atomic smoke. It is dedicated to the analysis of Arsenic(As), Mercury(Hg), Cadmium(Cd), Germanium(Ge), Selenium(Se), Lead(Pb), Bismuth(Bi), Tellurium(Ti), Tin(Sn), Zinc(Zn), Antimony(Sb) elements.
| Product Name | PF7
|
| Product Description | PF7 Atomic Fluorescence Spectrophotometer is an excellent element analysis instrument which has the advantages of atomic absorption spectrophotometer and atomic emission spectrophotometer but overcomes their shortages in techniques.
The instrument has good accuracy, high sensitivity, simple structure, small volume, and easy operation. It is designed to test elements that could easily form hydride, gaseous constituents or deoxidizes to atomic smoke. It is dedicated to the analysis of Arsenic(As), Mercury(Hg), Cadmium(Cd), Germanium(Ge), Selenium(Se), Lead(Pb), Bismuth(Bi), Tellurium(Ti), Tin(Sn), Zinc(Zn), Antimony(Sb) elements. |
| Features | Light source
High intensity hollow cathode lamps for improved sensitivity and stability. Pre-aligned lamp assemblies for trouble free installation. All lamps are uniquely data coded offering important information to the PF Win operating software. Up to 3 lamps can be installed for simultaneous analysis. Double beam optical system to eliminate drift from the light source and the detector. Shielded optical design greatly reducing light interference. Enhanced signal to noise ratio for increased analytical sensitivity. Unique optical configuration for increased Fluorescence intensity. High Quantum Solar Blind detector fitted as standard to ensure optimum stability. Atomiser System High precision quartz tube designed for optimum performance, durability and long life. Adjustable height control for improved optimisation. Integrated 2 stage, fully sealed, fume exhaust system to decontaminate toxic elements and pollution. Gold Mesh fitted to the chimney removes any mercury pollutant. Hydride Generator Integrated continuous flow Hydride System. Gas pressure sampling offers maintenance free operation. Online auto dilution and multiple auto purge by gas driven sequential injection system. Fully sealed reservoir bottles for extended solution life. New design Gas Liquid Separator with magnetic stirring for improved repeatability of analytical results. Liquid Separator cooled directly by specially designed Peltier system to remove unwanted water in the formed hydride and greatly reduce fluorescence quenching thus increasing the sensitivity. Unique high volume reagent storage positioned outside of the instrument to reduce contamination. Connection of carrier and reducer liquids to instrument using long life chemical resistant FEP tubing. Electronic Control High technology electronics and PCB components. PF Win software offers full control of PF7 instrument and accessories via Windows operating system Full GLP version available for multiuser group management and log record,QC functions, online data sharing self diagnostics, result and resource management. The Electronic Peltier is used as a condensing device and offers direct contact cooling for the gas-liquid separator, minimizing the water content of the hydride. This greatly reduces the signal scattering interference and quenching which improves the detection sensitivity. Safty Design Harmful gas exhaust is emitted via the exhaust hood. The relatively high density harmful elements are absorbed by a special reagent bag minimizing harmful gas inhalation. A fully enclosed waste bottle avoids volatile of acid and waste gas, reducing environmental pollution and laboratory personnel injuries. Inward optical design avoids ultraviolet light damage to personnel. Upgrad to Speciation Analysis The connection with speciation analysis units is simple and facilitates the upgrading of speciation analysis for elements such as As, Se, Hg, Sb. |
| Principle | The gas source provides a constant pressure environment for a constant liquid supply and solenoid valve control. The gas pressure takes up the sample (in sample loop) and the KBH4 (in reaction bottle) into the gas liquid separator which reacts and produces hydride gas (or atomic vapor). The Hydride gas enters the atomizer and is atomized, which produces fluorescence light. The detector receives intensity from the fluorescence light which then transmits a signal to the processor. The processed result is seen at the software on the PC system.
The instrument measures characteristics of the radiation exposure in the measured elements of atomic vapor, around the electronic ground state atoms that are excited to be high-energy state. Because the high-energy electronic state of the atoms become unstable, they return to a low energy state, The external radiation is measured by Fluorescence which is used for the quantitative analysis. The fluorescence intensity and the concentration of elements following the relations: In the formula:
If = ÖI 0(1− e −KëLN )5 If ———atomic fluorescence intensity; Ö ———atomic fluorescence quantum efficiency; I 0 ———Source radiation intense Kë ——–Peak absorption in wavelength ë ; L———-Light-absorption; N———Units within the length of the ground state atoms
The atomic fluorescence morphological analyzer is a specialized instrument used to test the valence states of elements such as arsenic, mercury, selenium, and antimony. This instrument is composed of a liquid chromatography separation system, an atomic fluorescence detection system, and a fluorescence interface section. The different valence state components of the tested elements have differences in physical and chemical properties, which can be reflected in the different retention times in the chromatographic column. This is the principle of the liquid chromatography separation system achieving separation of different valence states. The interface device brings the separated components of the tested elements with different valence states and other reagents participating in the hydride reaction into the reaction pipeline through the liquid transport equipment to achieve chemical reactions. Additionally, in the device, some organic valence state elements that cannot directly undergo hydride reactions or have low reaction efficiency can be transformed into inorganic valence state elements that can undergo hydride reactions through an online UV digestion device. The atomic fluorescence detection system quantitatively converts the tested elements into detectable spectral signals, and the data processing system detects and records these data while performing corresponding data processing. |
| Application | Food & Beverage production and quality control:Application to detection aquatic products, seafood, meat, alcohol, oral liquid, tea, dairy products, etc.
Epidemic prevention and sanitation:Trace sample testing for medicine and health, clinical analysis, disease control analysis, human biochemical index analysis, metabolite analysis, drug analysis, etc. Agricultural monitoring:applied to agriculture to complete the detection of grain, seeds, vegetables, soil, pesticides, etc. Environmental monitoring & control:applied to the determination of water quality, atmosphere, rainfall and soil and other pollutants. Life sciences:can be used to detect trace elements in human blood, hair, urine and tissues. Metallurgical: application in geological prospecting survey, detailed investigation and anomaly evaluation. Universities and colleges:Detection for element content determination, teaching demonstration, student experiments, scientific research, etc. Speciation analysis Chemical forms refer to the actual forms in which elements exist as ions or molecules. It includes several aspects such as the valence state, compound state, inorganic state, organic state, combined state, and structural state of the element. Taking arsenic and mercury as examples, there are various forms of arsenic and mercury in nature. Arsenic forms include inorganic arsenic, organic arsenic, various arsenic sugars, and organic arsenic acids and their derivatives. Mercury mainly exists in the forms of elemental mercury, inorganic mercury, and organic mercury. The common forms of arsenic include arsenite (As(III)), arsenate (As(V)), monomethylarsonic acid (MMA), dimethylarsinic acid (DMA), arsenobetaine (AsB), arsenocholine (AsC), and arsenosugar (AsS). The table below shows the median lethal doses (LD50) of these forms in experimental mice, indicating that arsenite (As(III)) and arsenate (As(V)) are the most toxic, while arsenobetaine (AsB), arsenocholine (AsC), and arsenosugar (AsS) can be considered essentially non-toxic. The common forms of mercury include inorganic mercury (Hg(II)), methylmercury (CH3Hg(I)), and ethylmercury (CH3CH2Hg). Methylmercury is much more toxic than inorganic mercury and has a strong affinity for living organisms. Additionally, inorganic mercury is easily accumulated and converted into methylmercury in biological systems. The famous Minamata disease is caused by methylmercury. Selenocysteine(SeCys) Selenite (Se IV) Selnomethionine (SeMet) Selenate (Se VI) Sb III Sb V |











.png)
.png)
.png)
.png)
.png)
.png)
.png)




























































.png)
1.png)





































.png)
1.png)
.png)
1.png)
.png)
1.png)
.png)
1.png)


















.png)
.png)